Abstract
Background
We showed earlier that prolonged treatment with a combination of azacitidine (DNMT1 inhibitor) and panobinostat (HDAC inhibitor) induced complete remission in a disseminated xenograft model of KMT2A rearranged AML (Gopalakrishnapillai et al., 2017, Leuk Res). Pretreatment with epigenetic drugs has been shown to induce chemo-sensitivity in some malignancies. However, studies on the use of epigenetic drugs in overcoming chemoresistance mediated by the bone marrow microenvironment are limited. The aim of this study was to determine the merit of incorporating epigenetic therapy with chemotherapy in AML treatment regimen and to identify the mechanism by which epigenetic drugs can chemosensitize AML.
Methods
AML cell lines and PDX lines were co-cultured with HS5 bone marrow stromal cells. Cell viability following drug treatment was assessed by flow cytometry. For determination of cell adhesion, violet proliferation dye stained AML cells were co-cultured with HS5 cells. Following treatment, non-adherent cells were removed by washes with phosphate buffered saline. The number of adherent AML cells was determined by flow cytometry. NSG-B2m mice were engrafted with MV4;11 cells and bled regularly to evaluate disease progression. Once engraftment was confirmed mice were assigned to treatment groups. Epigenetic therapy constituted azacitidine and panobinostat (2.5 mg/Kg each; Qd5). Chemotherapy constituted cytarabine (50 mg/Kg; Qd5) and daunorubicin (1.5 mg/Kg; Qd3). Mice were euthanized when they reached predetermined experimental endpoints. All animal studies were approved by the Institutional Animal Care and Use Committee.
Results
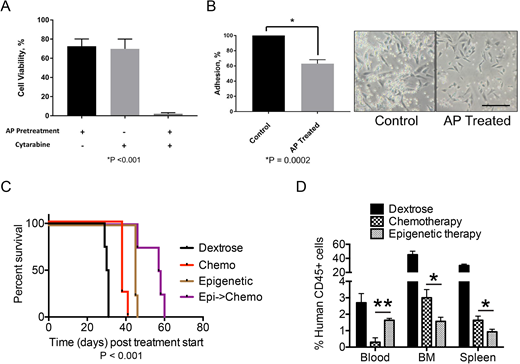
AML cells in co-culture with HS5 bone marrow stromal cells were less sensitive to chemotherapeutics cytarabine and daunorubicin compared to AML cells in monoculture. This chemoprotection was not achieved when AML cells were cultured in HS5 conditioned media or in Transwell inserts suspended over HS5 monolayers, suggesting that direct cell-to-cell contact was required. MV4;11 cells exposed to epigenetic drugs or cytarabine alone retained 70% viability. Pre-treatment with the epigenetic drug combination of azacitidine and panobinostat prior to cytarabine exposure greatly reduced cell viability in MV4;11 cells harboring KMT2A fusion (Fig. 1A). Similar chemo-sensitization was observed when four distinct KMT2A rearranged PDX lines were used ex vivo. This sensitization was accompanied by reduced binding of AML cells to HS5 cells following treatment with epigenetic drugs (Fig. 1B).
We evaluated the efficacy of the epigenetic therapy and chemotherapy combination in disseminated xenograft models of pediatric AML. NSG-B2m mice transplanted with MV4;11 cells via the tail vein were randomly assigned to four groups - 1) vehicle, 2) epigenetic therapy 3) chemotherapy (cytarabine + daunorubicin), and 4) epigenetic therapy followed by chemotherapy. The mice receiving the epigenetic therapy and chemotherapy combination survived significantly longer than mice treated with any other condition (Fig. 1C).
To assess the leukemic cell distribution in peripheral blood, bone marrow and spleen following treatment, a cohort of mice receiving epigenetic therapy alone or chemotherapy alone were euthanized a day after treatment concluded. The percentage of leukemic cells in bone marrow and spleen was lower in mice treated with epigenetic therapy than those treated with chemotherapy, consistent with the survival data. Surprisingly, the peripheral blood counts were significantly higher in mice receiving epigenetic drug combination. These results together with our in vitro data indicate that epigenetic therapy induces mobilization of AML cells to the blood stream. This increased availability of AML cells may promote enhanced sensitivity to chemotherapy.
Conclusion
Our data suggest that direct cell-to-cell contact plays a major role in mediating chemoprotective effects of the bone marrow microenvironment. These chemoprotective effects can be overcome by pretreatment with epigenetic drug combination azacitidine and panobinostat. Epigenetic drugs interfere with AML cell interactions with the microenvironment and dislodge AML cells from their protective niche. Thus, mobilization of AML cells to peripheral blood is a potential mechanism of chemo-sensitization mediated by epigenetic drugs.
Kolb:Servier: Membership on an entity's Board of Directors or advisory committees; Roche- Genentech: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal